Biohybrid Neural Interfaces
Overview
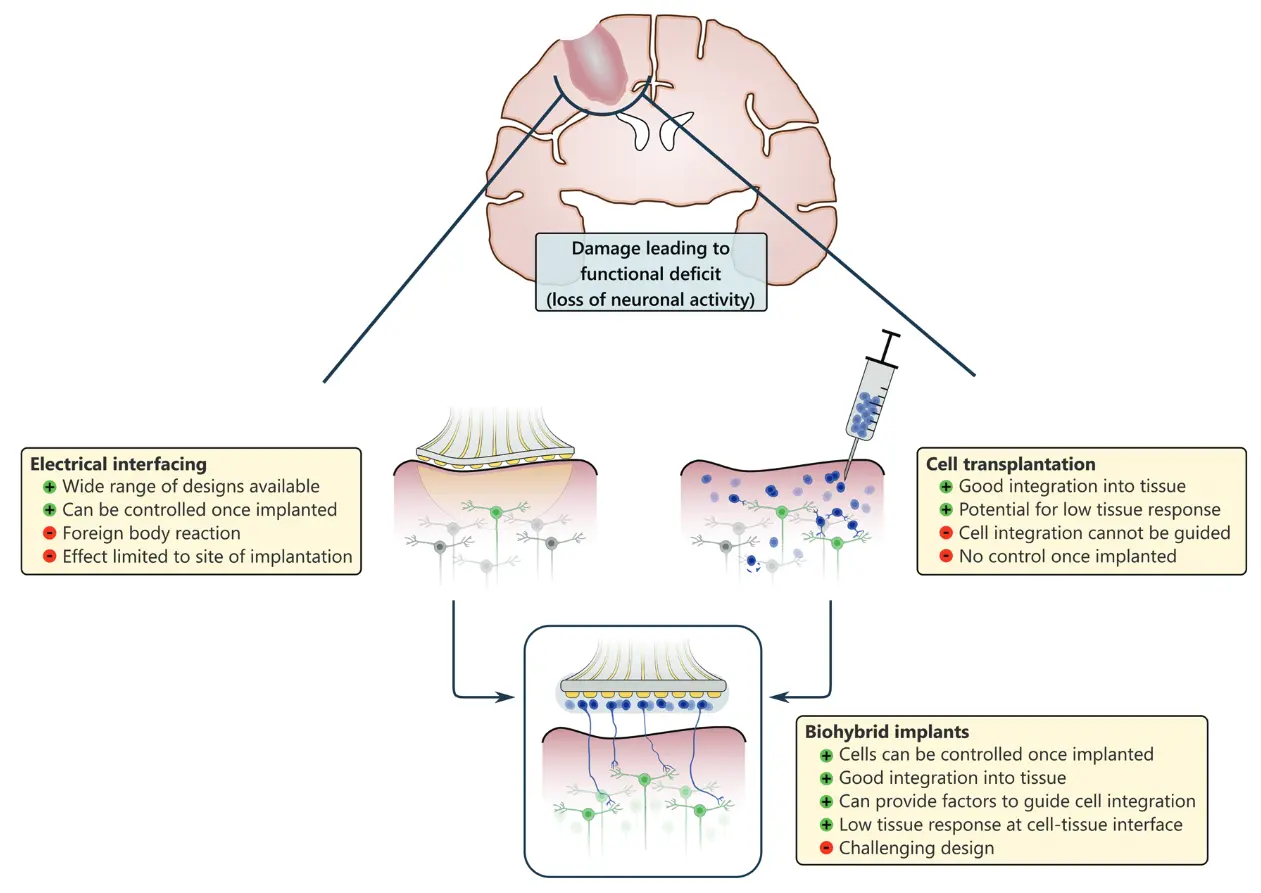
Recent advances in neural interface technology have enabled the development of biohybrid devices that integrate living cells with electrode-based platforms to restore function. Electrical stimulation supports neuronal regeneration in both the central and peripheral nervous systems, while living cells create pro-regenerative microenvironments that can be further modulated by targeted electrical cues. Following implantation, these cells integrate with host tissue to form a bioactive interface that reduces electrode degradation associated with the foreign body response. For long-term efficacy, biohybrid systems must maintain stability and biocompatibility, ensuring that neither the materials nor the cellular components elicit cytotoxic or adverse immune responses. Compared with conventional peripheral nerve interfaces, biohybrid implants demonstrate superior tissue integration, reduced axonal die-back and immune rejection, and enhanced functional regeneration.
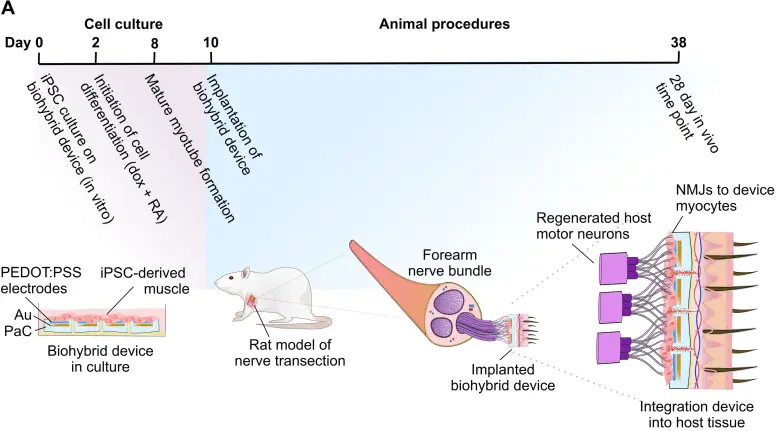
Prior research in our laboratory has validated the effectiveness of biohybrid architectures for motor function recovery through the incorporation of iPSC-derived muscle cells onto multielectrode array (MEA) platforms. These investigations demonstrated that biohybrid neural interfaces facilitate axonal extension, improve electrophysiological signal fidelity, and preserve long-term stability at the junction between electrodes and biological tissue.
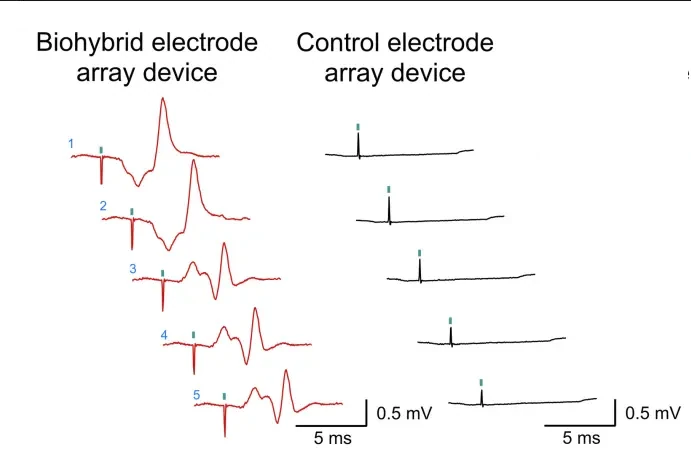
Cells in biohybrid devices act as biological amplifiers by transducing small extracellular nerve signals into larger bioelectrical responses through ion channel dynamics. Incoming signals depolarize the cell membrane, triggering voltage-gated Na⁺ and Ca²⁺ channels that generate amplified action potentials. This ionic flux increases the local extracellular potential, improving signal amplitude and signal-to-noise ratio. Furthermore, intercellular coupling via gap junctions can synchronize activity, further enhancing signal propagation. Such active biological processes enable detection of subtle neural signals that inert electrodes alone often fail to resolve.
A. E. Rochford, A. Carnicer-Lombarte, V. F. Curto, G. G. Malliaras, D. G. Barone, When Bio Meets Technology: Biohybrid Neural Interfaces. Adv. Mater. 32, 1903182 (2020). DOI:10.1002/adma.201903182
Amy E. Rochford et al. Functional neurological restoration of amputated peripheral nerve using biohybrid regenerative bioelectronics. Sci. Adv. 9, eadd8162 (2023). DOI:10.1126/sciadv.add8162
A. Carnicer-Lombarte, G. G. Malliaras, D. G. Barone, The Future of Biohybrid Regenerative Bioelectronics. Adv. Mater. 37, 2408308 (2024). DOI:10.1002/adma.202408308
Y.-L. Yu, J. Muzaffar, V. Philips, et al. “ Current Developments and Challenges in the Field of Biohybrid Neural Interfaces—A Scoping Review.” Adv. Electron. Mater. 11, no. 19 (2025): e00259. DOI:https://doi.org/10.1002/aelm.202500259